
Battery Technology Terms
Welcome to our comprehensive glossary of Battery Technology Terms. Here, you’ll learn about AkkuFresh® battery foil, battery performance terms, and battery charging explained. Whether you’re a tech enthusiast or a professional, this guide will help you understand key concepts in battery technology.
What is AkkuFresh®?
AkkuFresh® battery foil improves battery performance. It speeds up charging, extends usable time, and slows down capacity loss. This innovative foil works with any portable device.
Key Benefits of AkkuFresh®
- Installs in seconds.
- Boosts the battery’s charging ability.
- Provides more talk time and longer stand-by time.
- Reduces charging time by up to 40%.
- Restores worn or deteriorated batteries to their original performance.
- Prevents further battery deterioration.
- Protects the battery from electrical variations by regulating and filtering the current.
- Extends the total battery lifespan by up to 30%.
- Saves money by reducing the need for frequent battery replacements.
Important Notes
- Do not cover the electrical contacts on devices or batteries.
- Cover at least 80% of the battery on one side (size 29 x 50 mm). Use multiple pieces for larger batteries.
- Optimal improvements appear after 5-10 charging cycles.
Technology Overview
AkkuFresh® battery foil uses ionXtra® Power Foil™, a nano-ceramic material developed through nanotechnology. It is designed for modern portable devices with high energy demands, such as smartphones with color displays, cameras, internet access, Bluetooth, and multimedia functions.
How AkkuFresh® Works
AkkuFresh® acts as a frequency modulator. It reflects the electromagnetic field in the battery at a higher frequency, causing crystal particles to vibrate faster. This process regenerates the battery to its original capacity and increases the flow of negative ions.
Lithium ions also move more efficiently, increasing tension. The performance of AkkuFresh® depends on factors like the type of mobile phone, battery age, status, external temperature, environmental conditions, and cellular network.
Learn More
For more information on battery technology terms, check out this research paper on battery technology.
What are Alkaline Batteries?
Alkaline batteries are non-rechargeable batteries often used in devices requiring heavy currents for long periods, such as CD players and radios. They deliver 50-100% more energy than traditional Carbon/Zinc batteries, making them popular in consumer applications.
What is an Alloy?
An alloy is a mixture of several distinct metals or a metal and a non-metal.
A type of generator used in automobiles to produce an electric current.
The quantity of electricity measured in ampere-hours (Ah) that may be delivered by a cell or battery under specified conditions. The Amp-hour capacity of a battery (or cell) is its most important figure of merit. It is defined as the amount of current that a battery can deliver for 1 hour before the battery voltage reaches the end-of-life point.
The electrode in an electrochemical cell where oxidation takes place. During discharge, the negative electrode of the cell is the anode. During charge, the positive electrode is the anode.
The electrical characteristics of a battery define how it will perform in the circuit and the physical properties have a large impact on the overall size and weight of the product that it will power. The key properties and specifications for Ni-Cd, Ni-MH, and Li-Ion will be presented for easy comparison.
A battery is composed of one or more cells, either parallel or series connected to obtain a required current/voltage capability (batteries comprised of series-connected cells are by far the most common).
Is a set of any number of (preferably) identical batteries or individual battery cells. They may be configured in a series, parallel or a mixture of both to deliver the desired voltage and current. The term battery pack is often used in reference to cordless tools, radio-controlled hobby toys, and battery electric vehicles.
A cylindrical cell design utilizing an internal cylindrical electrode and an external electrode arranged as a sleeve inside the cell container.
The “c” rate is a current that is numerically equal to the A-hr rating of the cell. Charge and discharge currents are typically expressed in fractions or multiples of the c rate. Discharge or charge current, in amperes, expressed in multiples of the rated capacity. For example, C/10 discharge current for a battery rated at 1.5 Ah is: 1.5 AH/I 0 = 150 mA (A cell’s capacity is not the same at all discharge rates and usually increases with a decreasing rate.)
The total number of ampere-hours or watt-hours that can be gained from a fully charged cell or battery under specified conditions of discharge.
A correction factor applied to the rating of a battery if discharged under C-rates different from the one rated.
The fraction of the fall capacity available from a battery under specified conditions of discharge after it has been stored for a period of time.
A primary battery (non-rechargeable) commonly used in low-drain consumer applications (i.e.: clocks, calculators, garage door openers, etc…). Available in the same sizes as Alkaline and Manganese Dioxide (“AA”, “AAA”, 9volt, “C”, “D”), the Carbon/Zinc is one of the most widely used dry primary batteries because of its low price and reliable performance.
The electrode in an electrochemical cell where reduction takes place. During discharge, the positive electrode of the cell is the cathode. During charge in a rechargeable battery, the negative electrode is the cathode.
A cell is an electro-chemical device capable of supplying the energy that results from an internal chemical reaction to an external electric circuit.
Cells within a battery pack that contain different capacity and voltage levels.
The stronger cells of a battery (several cells connected in a series) impose a voltage of reverse polarity across a weaker cell during a deep discharge.
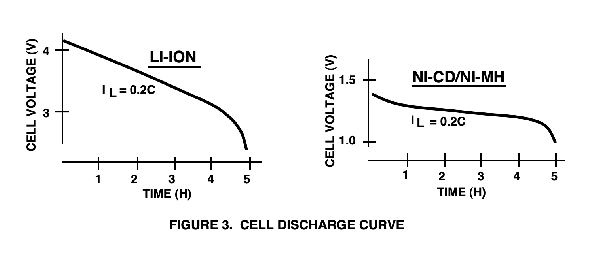
The voltage provided to power the load is obviously very important: The Ni-Cd and Ni-MH batteries have a 1.25V nominal cell voltage (their discharge voltages are generally assumed to be identical).
The Ni-Cd/Ni-MH cell voltage is only about one-third of the nominal 3.6V provided by a Li-Ion cell (see Figure 3), which means a designer is required to use three series-connected Ni-Cd or Ni-MH cells to equal the voltage of a single Li-Ion cell.
However, Figure 3 also shows the biggest advantage of Ni-Cd and Ni-MH batteries: their discharge curve is extremely flat, closest to an ideal battery.
This important difference between the battery types means that Ni-Cd and Ni-MH cells are well suited for use with linear regulators, but Li-Ion batteries require switching converters to obtain good energy conversion efficiency in the power supply.
The conversion of electrical energy, provided in the form of electrical current from an external source, to restore the chemical energy in a cell or battery.
A technique for effectively terminating the charging of a rechargeable battery.
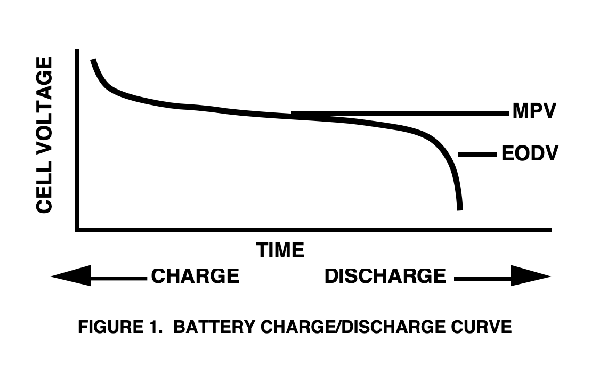
The measured terminal voltage of any battery will vary as it is charged and discharged (see Figure 1).
The MPV (mid-point voltage) is the nominal voltage of the cell during charge or discharge. The maximum and minimum voltage excursion from the nominal value is an important design consideration: a “flatter” discharge curve means less voltage variation that the design must tolerate.
When peak charged, the actual cell voltage will be higher than the MPV. When nearing the EODV (end of discharge voltage) point, the cell voltage will be less than the MPV. The EODV is sometimes referred to as the EOL (end of life) voltage by manufacturers.
Charge batteries with a DC current, positive terminal to positive terminal. The voltage must be greater than the battery or battery pack voltage. The current must be limited and cut off when the battery is fully charged.
Full charge is sensed either by a rise in voltage, a rise in resistance or a rise in temperature.
Battery chargers are designed to supply a suitable current for the batteries with which they were designed to work.
More sophisticated chargers will switch to a trickle when they sense the battery is fully charged. This is fine for Lead-Acid batteries but NiCd and NiMh should not be left on maintenance’charge for more than a few days.
The charging current is one eighth of the Ah (in Amps) or mAh (milliamps) rating of the battery. A full charge takes about 1.4 times the label capacity.
e.g. NiMh Battery 1800mAh capacity
charging current 1800 divided by 8 = 225 mA
charging time 1800 divided by 225 x 1.4 = 11.2 hours
The potential or voltage of a battery when it is discharging or charging.
A process that utilizes a series of heavy discharges and recharges on a battery to assure optimal performance.
A battery discharge regime with which the current is drawn during discharge. Discharge remains constant.
A battery discharge regime where the current during discharge increases as the battery voltage decreases.
A battery discharge regime where the resistance of the equipment load remains constant throughout discharge.
A constant-current charger is a circuit that charges a battery by sourcing a fixed current into the battery, regardless of battery voltage.
A constant-voltage charger is a circuit that recharges a battery by sourcing only enough current to force the battery voltage to a fixed value.
A test in which a battery is discharged to a prescribed point voltage without interruption.
The amount of electricity transported by a current of one ampere flowing for one second.
The rate at which (a volume of) electricity moves through a (pipe) conductor. Measured in Amps.
The potential is measured in (height) volts.
An inert structure of high electrical conductivity used to conduct current from or to an electrode during discharge or charge.
The current per unit active area of the surface of an electrode.
The current withdrawn from a battery during discharge.
A charger that keeps the charge current constant during the charging process but allows the voltage to fluctuate (typically used on NiCd and NiMh chargers).
The battery voltage at which the discharge is terminated. The cutoff voltage is specified by the battery manufacturer and is generally a function of the discharge rate.
A sequence where a charged battery is discharged and recharged.
The number of cycles that are available under specified conditions from a secondary battery before it fails to meet specified criteria relating to performance.
The positive and negative plates are rolled up and placed into a cylindrical container (as opposed to stacking the plates in a prismatic cell design).
The ratio of the quantity of electricity (usually in ampere-hours) removed from a battery to its rated capacity.
The opposite of absorption, when the material retained by a medium or another material is released.
Electrical current that flows in one direction only. Batteries produce direct currents as the current flows from a negative to a positive source.
The conversion of the chemical energy of a battery into electrical energy, and the withdrawal of the electrical energy into a load.
The rate, usually expressed in amperes, at which the electrical current is taken from the battery.
Never burn any battery, it may explode, it will release caustic electrolytes, and it may distribute harmful metal oxide particles.
Batteries containing silver, cadmium and mercury must undergo special disposal or may be returned.
Lithium batteries containing more than 0.5grams of Lithium metal present a special hazard.
Alkaline, NiMh, Zinc-Air, small Li-ion and Lithium coin cells, and conventional torch batteries can be put in domestic waste.
The current withdrawn from a battery during discharge.
A cell with immobilized electrolyte. The term “dry cell” is often used to refer to the Leclanche cell.
The operating regime of a battery including factors such as charge and discharge rates, depth of discharge, cycle duration, and length of time in standby mode.
Discharge or charge power, in watts, expressed as a multiple of the rated capacity of a cell or battery that is expressed in watt-hours. For example, the E/10 rate for a cell or battery rated at 17.3 watt-hours is 1.73 watts. (This is similar to the method of calculating the C-Rate.)
The movement of electrons along a conductor.
The weight of a substance that is deposited at an electrode when the quantity of electricity which is passed is one coulomb.
The site, area or location at which electrochemical processes take place.
The raised positive pip on a torch battery is connected to the battery cathode; the flat end is connected to the negative anode.
In a Lead-Acid battery (car battery), Lead is the anode and the acid (sulfuric acid, battery acid) is the electrolyte. Lead Dioxide is the cathode.
In a NiCd (Nickel-Cadmium) battery, Nickel is the anode and Cadmium the cathode.
The medium which provides the ion transport mechanism between the positive and negative electrodes of a cell.
Negatively charged particle that orbits the nucleus of an atom.
The prescribed voltage at which the discharge (or charge, if end-of-charge voltage) of a battery may be considered complete.
The output capability of a cell or battery, usually expressed in watt-hours.
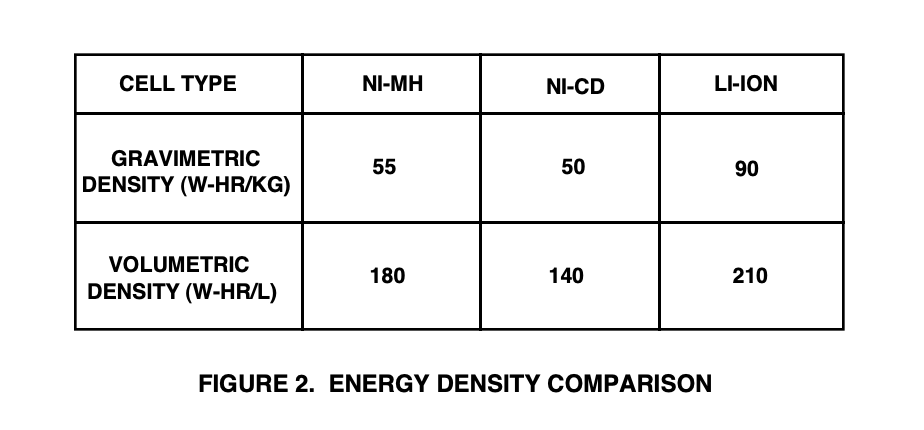
The ratio of the energy available from a battery to its volume (Wh/L) or weight (Wh/kg).
The energy density of a battery is generally expressed in two ways (see Figure 2):
The gravimetric energy density of a battery is a measure of how much energy a battery contains in comparison to its weight, and is typically expressed in Watt-hours/kilogram (W-hr/kg).
The volumetric energy density of a battery is a measure of how much energy a battery contains in comparison to its volume and is typically expressed in Watt-hours/liter (W-hr/l).
The measured cell voltage at the end of its operating life is called the EODV, which stands for End of Discharge Voltage (some manufacturers refer to this as EOL or End of Life voltage).
ESR (Equivalent Series Resistance) is the internal resistance present in any cell that limits the amount of peak current it can deliver.
Typical fast charge time for a NiCd is 1 to 3 hours. The fast-charger detects the state of charge and switches to trickle charge when full-charge is reached.
“Fast” charge (usually defined as a 1-hour recharge) requires more complex charging circuitry (again raising the system cost) but gives the customer faster charging time (a very attractive selling point).
The typical Ni-Cd or Ni-MH fast charger simply pumps current into the battery and waits for the battery to signal when it’s had enough. Because of the possibility of battery damage and user safety hazards, fast-charge systems must be designed to accurately monitor battery parameters like cell temperature and voltage. In addition, most have backup timers for a fail-safe cutoff of the high current charge applied to the battery.
The use of batteries which are charged by an application to be ready for use if the primary power to the application fails. Also called standby or backup.
Similar to trickle charge. Compensates for self-discharge on an SLA battery.
Discharging a cell in a battery, by the other cells or an external power source, below zero volts into voltage reversal.
A battery that produces an electric current from mechanically or continually replaced electrodes, e.g. hydrogen/ oxygen cells proposed for electric cars. Not generally available off-the-shelf.
Instead of burning the fuel to generate heat, the fuel cell generates electricity.
Device used for cutting off an electrical current in the event of emergency or under hazardous conditions.
The emission of gas from one or more of the electrodes in a cell. Gassing commonly results from local action (self-discharge) or from electrolysis of water in the electrolyte during charging.
The ratio of the energy output of a cell or battery to its weight (Wh/kg). This term is used interchangeably with the term ‘specific energy’.
The gravimetric energy density of a battery is a measure of how much energy a battery contains in comparison to its weight.
Connection to the earth or some conductor which takes the place of the earth.
Waste which is classified as “hazardous” (i.e.. potentially harmful to the environment) by the government.
The standard unit of frequency. A frequency of one complete cycle per second is a frequency of one hertz.
A discharge rate, in amperes, of a battery which will deliver the specified hours of service to a given cutoff voltage.
A device used to measure the specific gravity of the electrolyte in a cell.
Used in relation to the battery’s internal resistance, a test during which a battery is subjected to alternate periods of discharge and rest according to a specified discharge regime.
The opposition exhibited by a circuit element (cell or battery) to the flow of an alternating current (AC) of a particular frequency as a result of resistance, induction and capacitance.
The opposition exhibited by a circuit element to the flow of a direct current (DC). In a cell, the internal resistance is the sum of the ionic and electronic resistance of the cell components.
When a battery is connected to a circuit to do work, the current in the circuit is in inverse proportion to the resistance of the circuit plus the internal resistance of the battery. This can lead to the batteries warming up.
ionXtra-Power® is a new exclusively developed and constantly improved nanoceramic material.
On the island of Hokkaido, in the 1950ies, a Nanotech researcher found a special stone while analysing the curative power of volcanic lakes, whose waters have measurable positive effects on human health. Due to these and subsequent research results, in 1965 the location of the stone has been added to the list of Japan’s National Heritage Sites. It was formed the basis of thought process of the ionXtra-Power® and AkkuFresh® foil research and development.
ionXtra® Power™ is a new exclusively developed and constantly improved nanoceramic material. As a result of more than 25 years of R&D and of using the opportunities of nanotechnology, a mineral combination of natural, microporose crystal particles was created with the name of ionXtra® Power™.
The AkkuFresh® foil is made by applying 2µ thin ionXtra-Power® material into a layer and creating IonXtra® Power™ foil, which is a special foil, part of the many structural ultra thin layers of AkkuFresh® product. AkkuFresh® are specifically created for modern portable E-devices with a large color display, camera, internet access, Bluetooth, games and enhanced multimedia functions, which require high energy. IonXtra® Power™ foil technology is answers the growing battery life demands of the E-device market.
A voltage drop associated with the electrical resistance (R) of a battery or current flow (I). A voltage drop is the product of the current (in amperes) and resistance (in ohms).
Still the most popular type of battery used today, its main application is for the automobile industry, although it has a growing number of other applications. Its advantages include low costs, high voltage per cell and good capacity life. Disadvantages include poor low temperature performance, a relatively heavy weight, and that it cannot be left in a discharged state for too long without being damaged. Related batteries: Absorbent Glass Matt (AGM) Gel, Gel Cell sealed lead acid.
Zinc-Carbon pre-Alkaline torch batteries. In the lofts of old houses often there are open topped glass jars that used to have a carbon and zinc rod and ammonium chloride solution as an electrolyte, to provide a wet cell battery that powered the door bell. 1.5 volts, still made as a dry battery.
One of the more recent rechargeable battery technologies, Li-Ion batteries can deliver 40% more capacity than comparably sized NiCd batteries and are one of the lightest rechargeable batteries available today. Li-Ion batteries are the batteries of choice in notebook computers, wireless telephones and many camcorder models. They are also one of the more expensive rechargeability technologies.
3.6 volts per cell, light weight, high capacity, expensive batteries.
Not that much difference in capacity for the same volume as NiMh, but significantly lighter.
Li-Ion batteries can be ‘Top Up’ charged- recharged at any convenient time, without waiting for the battery to be completely discharged. About storage: store them as they are and allow them to self discharge slowly. Recharge them fully again when you want to use them.
A battery technology similar to Lithium-Ion.
Current “lithium-polymer” batteries are technically “Lithium Ion Polymer”, since pure lithium-polymer batteries are not yet commercially viable. Pure lithium-polymer batteries, once available, will theoretically provide considerable benefits over current battery technologies.
Today’s lithium-ion polymer batteries perform similarly to lithium-ion batteries, but can be made much thinner – as thin as 1mm.
A primary battery (non-rechargeable) that is quickly entering mainstream electronic design, particularly in consumer, portable equipment and non-volatile memory back-up applications where small size, long life and low costs are the primary requirements. Lithium batteries have superior cold temperature performance and a shelf life of 5-10 years.
The 3rd element, after Hydrogen and Helium. This position at one of the extremes gives it some interesting properties. Very different types of batteries are made containing Lithium; some are rechargeable some are not; some are safe, some are not.
Most of the lithium batteries you’ll see are in coin/button cell form. Coin cells are small discs, often Lithium cells are used (3V) but Alkaline, zinc air, and manganese are also used (1.5V).
They are very small and very light, great for small, low-power devices. They’re also fairly safe, have a long shelf life, and are fairly inexpensive per unit. However, they are not rechargeable and have high internal resistance (which is what makes them fairly safe if there’s only one or two in use) so they can’t provide a lot of continuous currents: 0.005C is about as high as you can go before the capacity is seriously degraded. However, they can provide a higher current as long as it’s ‘pulsed’ (usually about a 10% rate).
One of the most popular coin cells in use right now is the CR2032 which is 20mm diameter x 3.2mm thick, provides 220mAh at 3V. Lithium coin cells can get as large as the CR2477 (24mm x 8mm) with a capacity of 1000mAh for $3.50
The only other lithium cell you’ll see around is the CR123, which is a 3V cell that is a bit thicker than a AA battery and a bit shorter too.
The discharge current provided by a battery, or drawn by a battery powered device.
Magnesium batteries are used in sea going safety equipment. They are made without an electrolyte. When immersed in sea water, the salty water acts as an electrolyte and they produce power. They have a good but finite shelf life even in humid conditions. Once activated they must be replaced.
A primary battery (non-rechargeable) similar to the alkaline battery though not as strong in total energy. Available in the same size as Alkaline and Carbon/Zinc (“AA”, “AAA”, “C”,”D”, 9volt), the Manganese Dioxide chemistry is noted for its ability to retain its charge while being stored at high temperatures and operates well at temperatures as low as -40C with little loss of capacity.
A phenomenon in which a cell or battery operated in successive cycles to the same, but less than full, depth of discharge temporarily loses the rest of its capacity at normal voltage levels.
Some rechargeable batteries are said to have a memory. If they are part-used and recharged before the whole charge is used up, they ‘remember’ this and next time will only use that part of their capacity. Therefore part of their capacity is lost. This is theory, it is much debated.
NiCd and NiMh batteries are said to suffer from the “memory effect”.
NiCd and NiMh batteries prefer complete cycles; full charge then use until empty, do not recharge before storage, allow them to self-discharge during storage.
In the real world, either of these batteries will accept less than the ideal and provided that they are recycled completely, full to empty, reasonably often they will put up with what comes in between.
NiMh batteries display less “memory effect” than NiCd.
Good batteries have been made via using Mercury. These are not in common use nowadays because of potential pollution. Silver-Oxide or Zinc-Air cells make good or superior alternatives.
Mercury is a chemical element with the symbol Hg and atomic number 80. It is commonly known as quicksilver and was formerly named hydrargyrum (/haɪˈdrɑːrdʒərəm/ hy-DRAR-jər-əm). A heavy, silvery d-block element, mercury is the only metallic element that is liquid at standard conditions for temperature and pressure; the only other element that is liquid under these conditions is the halogen bromine, though metals such as caesium, gallium, and rubidium melt just above room temperature.
Mercury occurs in deposits throughout the world mostly as cinnabar (mercuric sulfide). The red pigment vermilion is obtained by grinding natural cinnabar or synthetic mercuric sulfide.
Mercury is used in thermometers, barometers, manometers, sphygmomanometers, float valves, mercury switches, mercury relays, fluorescent lamps, and other devices, though concerns about the element’s toxicity have led to mercury thermometers and sphygmomanometers being largely phased out in clinical environments in favor of alternatives such as alcohol- or galinstan-filled glass thermometers and thermistor- or infrared-based electronic instruments. Likewise, mechanical pressure gauges and electronic strain gauge sensors have replaced mercury sphygmomanometers.
Mercury remains in use in scientific research applications and in amalgam for dental restoration in some locales. It is also used in fluorescent lighting. Electricity passed through mercury vapor in a fluorescent lamp produces short-wave ultraviolet light, which then causes the phosphor in the tube to fluoresce, making visible light.
Mercury poisoning can result from exposure to water-soluble forms of mercury (such as mercuric chloride or methylmercury), by inhalation of mercury vapor, or by ingesting any form of mercury.
A mercury battery (also called mercuric oxide battery, mercury cell, button cell, or Ruben-Mallory[1]) is a non-rechargeable electrochemical battery, a primary cell. Mercury batteries use a reaction between mercuric oxide and zinc electrodes in an alkaline electrolyte. The voltage during discharge remains practically constant at 1.35 volts, and the capacity is much greater than that of a similarly sized zinc-carbon battery. Mercury batteries were used in the shape of button cells for watches, hearing aids, cameras, and calculators, and in larger forms for other applications.
For a time during and after World War II, batteries made with mercury became a popular power source for portable electronic devices. Due to the content of toxic mercury and environmental concerns about its disposal, the sale of mercury batteries is now banned in many countries. Both ANSI and IEC have withdrawn their standards for mercury batteries.
An inter-metallic compound or alloy in which hydrogen has been absorbed. Also, the negative electrode in a nickel-metal hydride battery.
Refers to battery capacity. A 1/1000th of an amp, e.g.: 1.0Ah = 1000mAh.
The voltage of a battery halfway through the discharge between the start of the discharge and the end voltage.
A terminal or electrode which has an excess of electrons.
One of the most proven and historically most widely used rechargeable batteries. Very dependable and “robust” but contain cadmium and have relatively low capacity when compared to other rechargeable systems. Very good high rate discharge capabilities make them very popular in high drain applications such as power tools.
Due to its Cadmium content this battery must be disposed of safely. NiCd batteries have gained a bad reputation for displaying the memory effect.
NiCd batteries prefer to be charged when they show a drop in power (to over-discharge a battery pack is to risk ‘voltage reversal’ of the weakest cell).
Store as they are, and recharge before use.
NiCd batteries self-discharge. They lose about 40% of their charge in 4 weeks.
NiCd batteries have a low internal resistance:
- they can deliver a high current,
- they don’t overheat easily in use,
- they can be charged quickly.
Used sensibly, they have a life span twice as long as that of NiMh or Li-ion batteries.
1.2 volts per cell, reasonably constant over the discharge cycle
A nickel-metal hydride battery (NiMH or Ni–MH) is a type of rechargeable battery. The chemical reaction at the positive electrode is similar to that of the nickel-cadmium cell (NiCd), with both using nickel oxide hydroxide (NiOOH). However, the negative electrodes use a hydrogen-absorbing alloy instead of cadmium. NiMH batteries can have two to three times the capacity of NiCd batteries of the same size, with significantly higher energy density, although much less than lithium-ion batteries.
They are typically used as a substitute for similarly shaped non-rechargeable alkaline batteries, as they feature a slightly lower but mostly compatible cell voltage, and are resistant against leaking and explosion.
The characteristic operating voltage or rated voltage of a battery.
A measure of resistance that causes one volt to produce a current of one ampere. The ohm is defined as an electrical resistance between two points of a conductor when a constant potential difference of one volt, applied to these points, produces in the conductor a current of one ampere, the conductor not being the seat of any electromotive force.
The difference in potential between the terminals of a cell when the circuit is open (the ‘no-load’ condition).
The process of discharging a cell or battery beyond its cutoff voltage and possibly into voltage reversal.
To force a current through a cell after all the active materials have been converted to the charged state, that is, continued charging after reaching 100 % charged status.
Term used to describe the interconnection of cells or batteries in which all the like terminals are connected together (positive to positive, negative to negative). Results in increased capacity (sum of the total). NiCd, NiMh and Lithium cells should not be connected in parallel.
The phenomenon by which a metal, although in conditions of thermodynamic instability, remains indefinitely un-attacked because of modified or altered surface conditions.
The maximum current that a battery can deliver is directly dependent on the internal equivalent series resistance (ESR) of the battery.
The current flowing out of the battery must pass through the ESR, which will reduce the battery terminal voltage by an amount equal to the ESR multiplied times the load current (V = I X R).
More importantly, the current flowing through the ESR will cause power dissipation within the battery that is equal to the ESR multiplied times the current squared (P = I2 X R). This can result in significant heating within the battery at high rates of discharge.
Both Ni-Cd and Ni-MH batteries have extremely low ESR values (well below 0.1W for a typical “AA” cell), which means that ESR is almost never a limitation for peak discharge current in these cell types.
The Li-Ion battery will typically have a higher ESR (compared to Ni-Cd or Ni-MH), but will probably not be a problem in most applications.
In electricity, the condition of being positive or negative.
The lowering of the potential of a cell or electrode from its equilibrium value caused by the passage of an electric current.
A terminal or electrode which has a shortage of electrons.
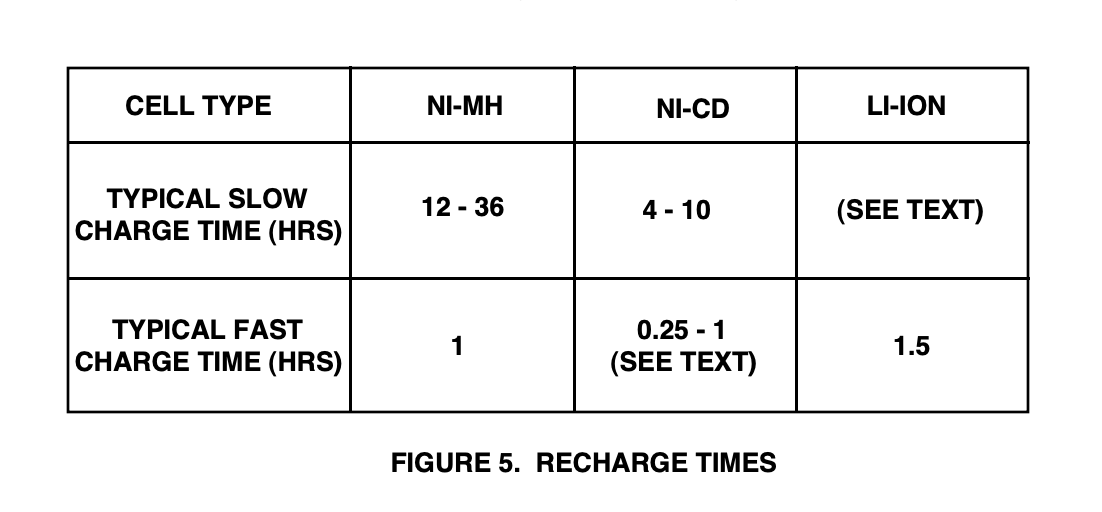
The amount of time that the typical consumer finds acceptable for battery recharging is highly variable and depends on the item being powered. Figure 5 shows the typical minimum charging times for slow and fast charging rates of the three battery types.

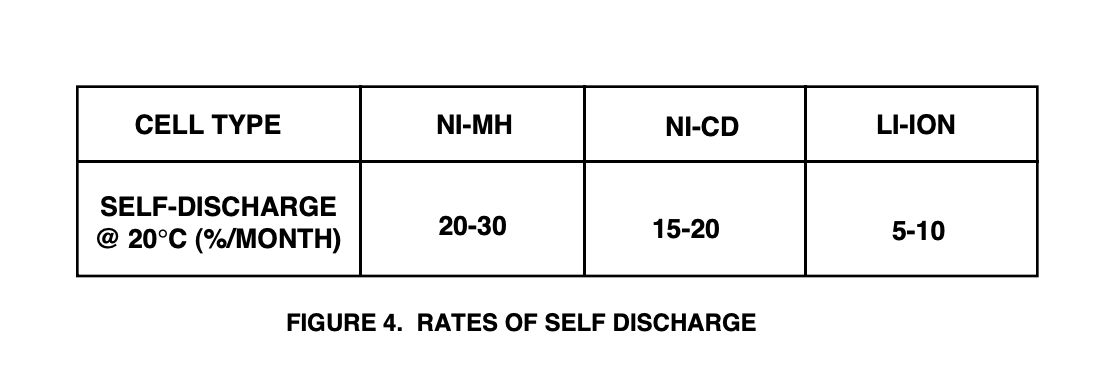
Self-discharge (which occurs in all batteries) determines the “shelf life” of a battery. Figure 4 shows typical self-discharge rates for the three chemistries, exact values will vary with the manufacturer.
In general, Li-Ion is the best of the lot, while Ni-Cd and Ni-MH are fairly comparable to each other. Ni-Cd is typically a little better than Ni-MH, but this may even out as Ni-MH manufacturing technology matures.
It is important to note that self-discharge is highly dependent on temperature, increasing as the battery temperature is increased.
Another unpleasant characteristic (I have heard voiced with respect to Ni-MH batteries used in cellular phones and laptop computers) is that the discharge rate is extremely non-linear. A battery that loses 30% in a month may lose 15 to 20% in the first few days (not good if you are taking a couple of spare batteries on a week-long trip, and you don’t want to carry the charging station).
A “Slow” charge is defined as a charging current that can be safely applied to a battery indefinitely without any kind of monitoring or charge termination method (it is sometimes referred to as trickle charging). A typical Ni-Cd battery will easily tolerate c/10, and some fast-charge Ni-Cd cells will accept up to c/3.
Ni-MH cells are not as tolerant of constant charging, as most will not handle a sustained charging current greater than c/40 (although one manufacturer advertises cells that are rated for c/10 trickle charge rate).
It is important to note that Li-Ion cells will not tolerate trickle charging at all after they are fully charged. If the current is continuously forced into a fully-charged Li-Ion cell (even a very minute current) the cell will be damaged. For this reason, Li-Ion cells are charged using constant-voltage (C-V) chargers, and not constant-current (C-C) chargers.
If a product is designed only for slow (overnight) recharging, a user may have to buy a second battery pack and keep it on “standby” charge (increasing the amount of money he has to spend).
The volumetric energy density of a battery is a measure of how much energy a battery contains in comparison to its volume.
Feedback
If you spot any errors in the translations or would like to provide feedback, please visit our Contact Us page.
We consider all feedback non-confidential and may use it on an unrestricted basis.
Date of Last Revision: December 1, 2024